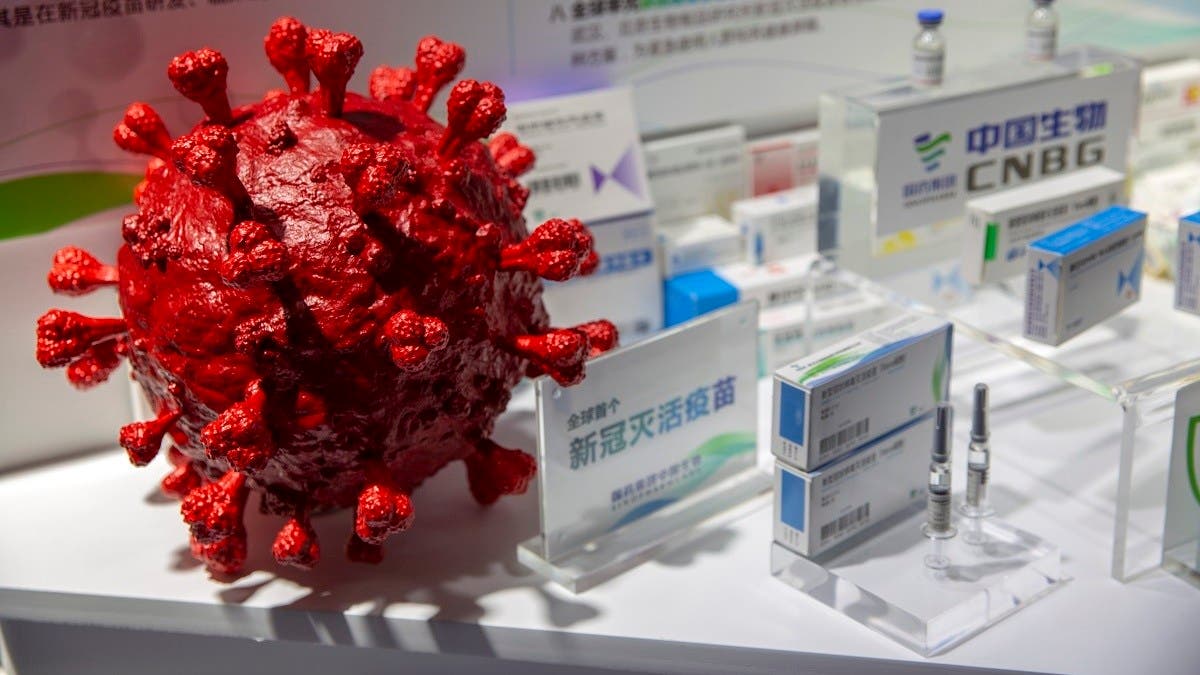
The United Arab Emirates has approved emergency use of Sinopharm’s protein-based COVID-19 vaccine and it will be available to the public as a booster dose starting January 2022, the health ministry said this week.
The Ministry of Health and Prevention approved the recombinant protein-based vaccine this week ahead of a planned roll-out next month.
For more coronavirus news, visit our dedicated page.
What is the vaccine?
The Sinopharm CNBG recombinant protein-based vaccine uses an S protein that surrounds the COVID-19 virus, which helps the body identify the virus and fight it in case of exposure. The technology “will help in the prevention of several variants,” the UAE’s National Emergency Crisis and Disaster Management Authority (NCEMA) tweeted on Tuesday.
“Sinopharm CNBG recombinant protein-based vaccine, as well as the other inactivated Sinopharm vaccine, are effective in producing antibodies, but the new protein-based vaccine could achieve more widespread protection to ensure higher effectiveness against variants.”
The technology is not new, as it has been used to produce ither vaccines such as the hepatitis B vaccine.
What are the side effects?
NCEMA has said that the effects are similar to other vaccines, including headaches, pain in the vaccinated area, and exhaustion with possible fever.
Who is eligible?
NCEMA said Sinopharm CNBG recombinant protein-based vaccine may be given to people over the age of 18 as a booster shot six months after receiving the Sinopharm vaccine. Individuals allergic to any of the vaccine components are exempted after undergoing evaluations conducted by the medical team. Pregnant women, nursing mothers, and those planning pregnancy within the next six months are exempted from the vaccine, but may receive other types of authorized vaccines.
Authorities are urging everyone to get a booster jab, with NCEMA saying: “Booster shots are among the key factors that help protect the health and safety of the community, as they play an effective role in boosting collective immunity.”
When was it approved?
The approval came this week following a UAE-based study that included individuals who were previously vaccinated with two doses of Sinopharm CNBG’s inactivated vaccine, the health ministry said.
How many COVID-19 vaccines have been administered so far?
The UAE continues to be a world leader in vaccination rates, with more than 22.5 million does provided so far. Around 91 percent of the population of some 10 million has been fully vaccinated.
Read more:
UAE approves Sinopharm’s protein-based vaccine as a booster dose
Abu Dhabi reimplements distance learning amid COVID-19 surge
UAE reports another rise in new daily COVID-19 cases

 World2 years ago
World2 years ago
 World2 years ago
World2 years ago
 Entertainment7 years ago
Entertainment7 years ago
 World7 years ago
World7 years ago
 Entertainment7 years ago
Entertainment7 years ago